Service
The measurement of voltage and current is essential in electrochemical analysis for determining chemical compounds in solution and their concentrations. Electrodes made of gold or platinum, in combination with reference electrodes such as Ag/AgCl , are often used for this application. A typical disadvantage of conventional measurement setups is the need for at least 20 ml of analyte, which requires large electrodes.
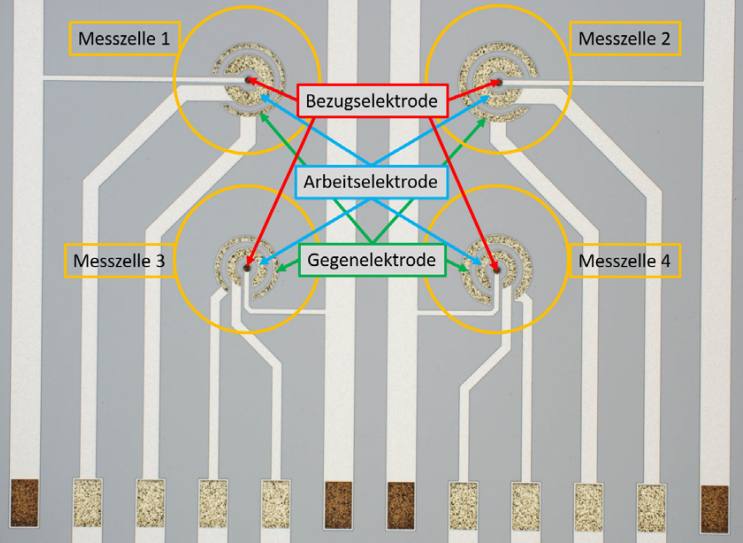
As a consequence, the electrodes can be expensive and have to be cleaned periodically with substantial effort. Additionally, contaminated electrodes might cause measurement errors, where the replacement of electrodes made of precious metals is costly and environmentally unfriendly. The analytic chips developed by IPMS can overcome these issues and are especially suited for potentiometry and voltammetry measurements. The chips, with an area of 5 x 5 mm2, offer different electrode geometries, sizes, and materials for the working, counter, and reference electrodes.
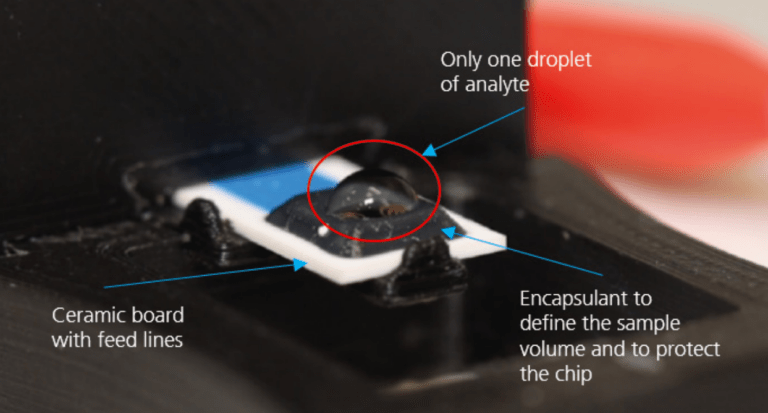
Precise electrode geometries and dimensions provide another advantage for electrochemical measurements. The chips with these electrodes are easy to handle and require only a very small amount of precious metal due to their resourceefficient fabrication. Analysis with the chips can be performed directly with a measuring adapter, or they can be bonded to a ceramic board. The size of the ceramic boards is 30 x 9.3 x 0.63 mm3 and the boards have six contact pads, allowing for the use of up to two individual 3-electrode measuring cells on one chip.
A sample size of one droplet ( ~20 μl) containing the analyte is sufficient. The setup is compatible with aqueous solutions (pH 2… pH 10), as well as with most organic solvents. Available electrode materials are platinum, gold, silver and copper, with thicknesses from 1 μm to 3 μm. Customized chips, sensors, electrode geometries, and electrode materials are possible, thereby covering a broad spectrum of applications.