The skin irritant potential of the materials can be determined depending on their use by OECD Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method or Method to Detect Skin Irritation of Medical Device Extracts using Reconstructed human Epidermis (RhE).
OECD Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method
The EpiDerm™ SIT (EPI-200-SIT, MatTek Corporation,) was developed and designed to predict skin irritation potential of the substances in the context of identification and classification of skin irritation hazard according to the EU classification system (R38 or no label). The test discriminates between irritants of category 2 and non-irritants.
This test is applicable to solids, liquids, semi-solids and waxes. The liquids may be aqueous or non-aqueous; solids may be soluble or insoluble in water. Whenever possible, solids should be ground to a fine powder before application. Moreover, when considering testing of mixtures, difficult-to-test chemicals (e.g. unstable), or test chemicals not clearly within the applicability domain described in the OECD TG 439 guideline, different considerations should be taken into account. These considerations are not needed when there is a regulatory requirement for testing of the mixture. However, due to the fact that mixtures cover a wide spectrum of categories and composition, and that only limited information is currently available on the testing of mixtures, this TG should not be used for this TG. Similar care should be taken in case specific chemical classes or physico-chemical properties are found not to be applicable to the current TG.
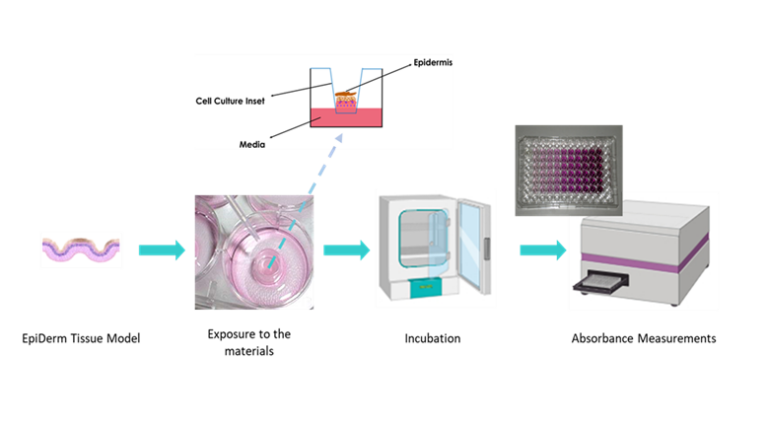
Briefly, the method consists of a topical exposure of the chemical to a reconstructed human epidermis (RhE) model for 1 h followed by a cell viability test (MTT assay). Cell viability is measured by dehydrogenase conversion of MTT [(3-4,5-dimethyl thiazole 2-yl) 2,5-diphenyltetrazoliumbromide], present in cell mitochondria, into a blue formazan salt that is quantitatively measured after extraction from tissues. The reduction of the viability of tissues exposed to chemicals in comparison to negative controls (treated with PBS) is used to predict the skin irritation potential. Optionally, other endpoints could be determined such as the release of IL-1α.
Method to Detect Skin Irritation of Medical Device Extracts using Reconstructed human Epidermis (RhE)
The test is designed to predict and classify the skin irritation potential of medical device extracts according to the requirements of ISO standards 10993-1:2009, 10993-5:2009, 10993-10:2010, and 10993-12:2012 using the RhE model EpiDerm™ (EPI-200) and parameters related to skin irritation by measuring the effect of the extracts on the viability of reconstructed human epidermis (RhE).
This test is applicable to a wide range of materials with different thickness such as film, sheet, tubing wall, slab, small moulded items, larger moulded items, elastomeric closures, powder, pellets, foam, non-absorbent moulded items, membranes and textiles.
A brief description of the procedure, the materials are first extracted with polar (0.9 % NaCl solution) and non-polar (sesame oil) solvent vehicles. Then, 100 μL of the extracts are topically applied to the EpiDerm™ (EPI-200, MatTek Corporation) tissues for 18 h. After the exposure, cell viability is determined by MTT assay. The reduction of the viability of tissues exposed to extracts in comparison to negative controls (treated with PBS) is used to predict the skin irritation potential. Optionally, other endpoints could be determined such as the release of IL-1α.