Result description
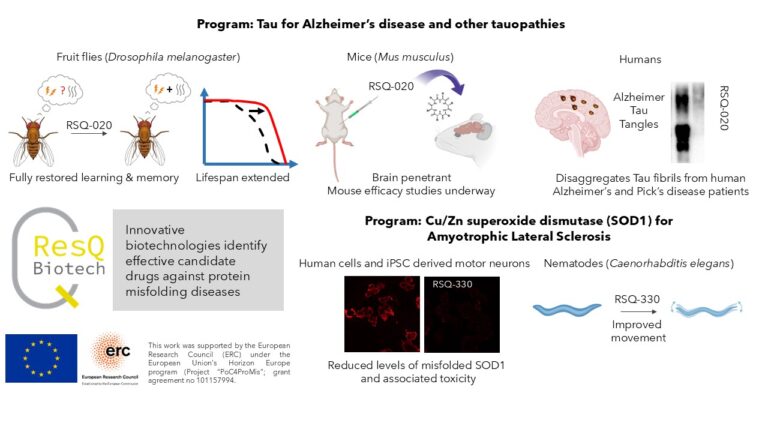
ResQ Biotech develops brain-penetrant cyclic peptides that directly reverse disease-driving protein misfolding. Our AD lead molecule RSQ-020 disaggregates Tau fibrils from human Alzheimer’s and Pick’s disease brains and, under therapeutic dosing, restores learning, memory and lifespan in Drosophila tauopathy models; mouse efficacy studies are in progress. In ALS, RSQ-330 rescues misfolded SOD1 and neurotoxicity in patient iPSC-derived motor neurons and improves movement in ALS models in the nematode Caenorhabditis elegans; in vivo mouse studies are being planned. We’re seeking private investments to complete preclinical development and initiate clinical development for our leading indications.
SOD1 (ALS): We identified the cyclic oligopeptide RSQ-330, which rescues misfolding, aggregation and the resulting neurotoxicity of multiple ALS-linked SOD1 mutants in human cell lines, patient iPSC-derived motor neurons, and an ALS C. elegans model. To our knowledge, this is the first chemical agent shown to rescue SOD1 misfolding in human patient-derived neurons.
Tau (AD/tauopathies): We identified the cyclic oligopeptide RSQ-020, which disassembles Tau fibrillar aggregates from human AD and tauopathy brains and disaggregates human Tau fibrils ex vivo from Tau-transgenic mouse brains. In a Drosophila tauopathy model, RSQ-020 fully restores lifespan, learning and memory under preventive and therapeutic dosing at 1–10 nM. In head-to-head tests vs EGCG (AD phase 2) and LMTX (AD phase 3), RSQ-020 outperformed and uniquely restored cognition. RSQ-020 is BBB-penetrant, reaches the mouse brain, and shows no detectable toxicity at pharmacologically relevant doses.
Influence: First-in-class, brain-penetrant cyclic peptides that reverse pathogenic protein misfolding in AD and ALS; in-vivo efficacy de-risks IND/CTA and opens a new modality for neurodegeneration targets.
Addressing target audiences and expressing needs
- Grants and Subsidies
- Collaboration
- Venture Capital
- Private Investments to complete preclinical development and advance toward IND/CTA readiness for clinical development.
- Co-development/licensing discussions with pharma/biotech active in Alzheimer’s, ALS, neurodegeneration and protein misfolding diseases.
- CRO/CDMO partners for mouse efficacy, GLP tox/ADME, LC–MS/MS bioanalysis, and GMP CMC; plus regulatory support (EMA/FDA scientific advice).
- Investor introductions for seed/Series A aligned with the regulatory path.
- Public or private funding institutions
- Research and Technology Organisations
- Private Investors
R&D, Technology and Innovation aspects
Current stage (RSQ-020): TRL-4 brain-penetrant cyclic peptide lead. Next steps: mouse efficacy; PK/PD + biomarkers; CMC scale-up to GMP drug substance/formulation; rat/dog GLP tox—advancing to TRL-5 with a CTA/IND-ready plan. Funding: seeking €3M co-investment now; €10–15M to reach TRL-6/IND.
We lead with platform and assets. Our hybrid model scales fast: (1) license molecules we develop and (2) provide Discovery-as-a-Service. This delivers near-term revenue from paid pilots, options and licenses while we capture long-term upside through clinical asset development. Horizontally, the platform scales by plug-and-play retargeting to new misfolded proteins; our living, ultra-large in-cell engine runs parallel campaigns at low marginal cost, moving from target to validated hits in weeks. Vertically, we scale through assets: advancing Tau programs toward IND/CTA creates inflection points for upfronts, milestones and royalties; the same modality extends to other tauopathies and protein-misfolding diseases. Capacity expands via CRO/CDMO partners while ResQ retains design, data and IP. Each program sharpens the playbook, reducing risk and accelerating the next deal. This is a capital-efficient path to multiple assets and durable revenues.
Replicability is built into both platform and assets. Our in-cell discovery engine runs on defined host strains and fully mapped, versioned plasmids/libraries whose composition is verified. SOPs with positive/negative controls let the same screens be rerun across sites with identical settings. Hits are confirmed through a fixed cascade: (i) biochemical readouts (insoluble-fraction immunoblot, Thioflavin T fluorescence), (ii) human cell systems (seeded-aggregation imaging, misfolded-protein immunostaining, flow cytometry; iPSC-derived neurons with soluble/insoluble fractionation and viability), and (iii) invertebrate models (Drosophila melanogaster and Caenorhabditis elegans). For all stages we lock outcomes and analysis plans before starting, use dose–response testing, independent repeats, and randomization/blinding. Protocols and raw-data packages transfer cleanly to CROs.
Sustainability rests on two pillars: durable results and a resilient business model. Scientifically, our platform uses standardized SOPs, shared controls, and a stepwise confirmation cascade (biochemical → human cells → in vivo) so findings are repeatable and extendable to new protein-misfolding targets. Each program adds reusable assays, biomarker readouts, and design rules that strengthen the next compounding know-how rather than restarting from zero. Commercially, we are not a single-asset bet. The same engine produces multiple assets across PMDs, while Discovery-as-a-Service, research licenses, and options create recurring, early revenue. Co-development and out-licensing de-risk later stages while retaining upside via milestones and royalties. Our bacterial, low-reagent workflows reduce cost and environmental footprint. Together, this enables a scalable, capital-efficient company that can thrive beyond any one indication or trial.
Result submitted to Horizon Results Platform by RESQ BIOTECH IDIOTIKI KEFALAIOUCHIKI ETAIREIA